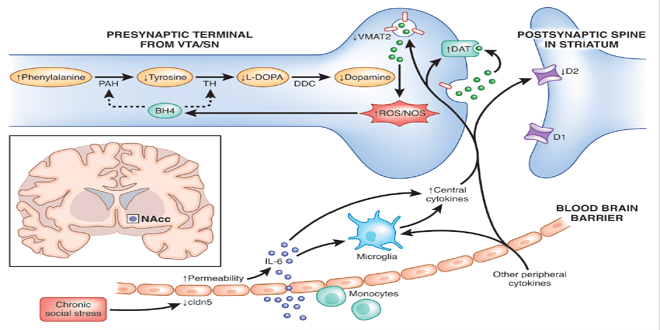
Ample data suggest an association between inflammation and inflammatory cytokines and the development of behavioral syndromes, including disorders of mood and anxiety. Accordingly, much attention has been paid to the mechanisms by which peripheral inflammatory processes access the brain and interact with pathophysiologic pathways relevant to behavioral regulation, including neurotransmitter metabolism, synaptic plasticity, and regional brain activity.
Access of Cytokines to the Brain
Cytokines are relatively large molecules that do not freely cross the blood-brain barrier (BBB). Nevertheless, studies have elucidated a number of routes by which cytokine signals can reach the brain, including humoral and neural routes as well as direct penetrance of the BBB by infiltrating immune cells.
Upon release into the peripheral circulation, cytokines can enter the brain through leaky regions in the BBB located in the circumventricular organs around the third and fourth ventricles. The circumventricular organs in mammals include the median eminence and adjacent neurohypophysis, organum vasculum lamina terminalis, subfornical organ, and the area postrema.
103 These brain regions are characterized by fenestrated capillaries where cytokines can enter brain parenchyma through passive diffusion and activate relevant cell types, including microglia. Circulating cytokines can also access the brain through binding to saturable transport molecules on endothelial cells that make up the BBB.101,104 Transport molecules for a number of cytokines have been described, including IL-1 and TNF-alpha.
Neurotransmitter Metabolism
small-molecule neurotransmitters. The monoamines, serotonin, norepinephrine, dopamine, and small-molecule neurotransmitters, glutamate, and gamma-aminobutyric acid (GABA), are well known to play key roles in the regulation of behavior and are all implicated in the pathophysiology and treatment of mood and anxiety disorders.
There is a rich literature in laboratory animals demonstrating that acute administration of cytokines or cytokine inducers such as LPS can profoundly alter neurotransmitter turnover.112 Fewer studies have been conducted in humans, but the data are consistent with the notion that chronic exposure to peripheral inflammatory cytokines can alter neurotransmitter metabolism in the brain in association with changes in behavior.
Synaptic Plasticity
While cytokines in the CNS provide trophic support to neurons and contribute to normal cognitive functions such as memory in laboratory animals, significant data indicate that in the context of excessive or prolonged activation, cytokine networks in the CNS can promote an interconnected suite of abnormalities that are increasingly thought to be relevant to the pathophysiology of depression, including diminished neurotrophic support, decreased neurogenesis, increased glutamatergic activation.
Whether as a result of an immune challenge or acute or chronic stress, increases in inflammatory cytokine production have been shown to contribute to decreased neurotrophic support and neurogenesis in brain areas important to behavior and cognition. For example, LPS administered peripherally produces cognitive impairment and increased hippocampal concentrations of TNF-alpha and IL-1, which are associated with decreased hippocampal expression of brain-derived neurotrophic factor (BDNF) and its receptor, tyrosine kinase-B.
as well as reduced hippocampal neurogenesis. In addition, blockade of CNS cytokine activity via administration of IL-1 receptor antagonist (IL-1ra) or transplantation of IL-1ra-secreting neural precursor cells into the hippocampus or the use of IL-1 receptor KO mice has been shown to prevent the effects of acute and chronic stress on behavior, cognition, neurotrophic factors, and neurogenesis. Of note, in vitro studies, have suggested that cytokine effects on neurogenesis are mediated in part by activation of NF-κB.
Last word
on the basal ganglia may ultimately support evolutionarily derived pressures to reallocate energy resources from environmental exploration to fighting infection and wound healing.